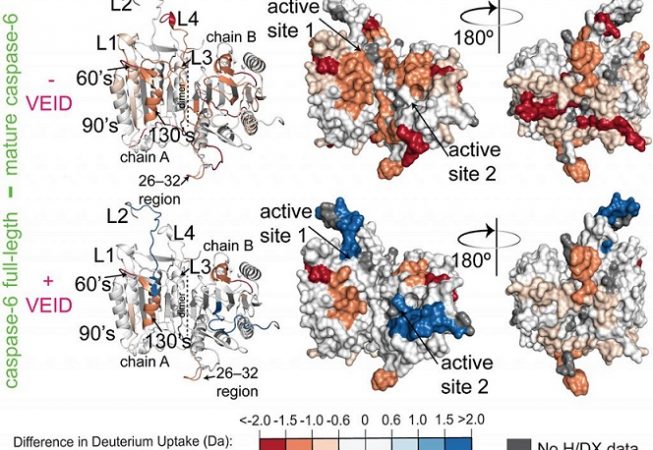
Caspase-6 is a dimeric cysteine aspartate protease involved in multiple biological pathways including apoptosis and neurodegeration. It is composed of prodomain (resi. 1-23), large subunit (resi. 24-179), intersubunit linker (resi. 179-193), and small subunit (resi. 193-293) for each monomer. Removal of the prodomain as well as intersubunit linker is a perquisite for caspase-6 maturation and then substrate binding. Multiple crystal structures of caspase-6 have been solved by the Hardy group and others.[1-3]However, these crystal structures lack structural-dynamic information. The Hardy Group has employed H/DX-MS technique to identify most important regions of caspase-6 upon substrate binding (here, VEID-cho). The H/DX data demonstrates that the active sites along with the dimer interface (consisting intersubunit linker) are highly exposed (colored red) to the solvent in the absence of VEID-cho. Moreover, drastic dynamic changes have been observed for these regions as unexposed (shifting from red to blue) to the solvent in the presence of VEID-cho. This structural-dynamic study will be helpful in identifying allosteric sites and therefore in designing potent as well as selective inhibitors of caspase-6.
Kevin B. Dagbay and Jeanne A. Hardy (2017) “Multiple proteolytic events in caspase-6 self activation impact conformations of discrete structural regions” PNAS 114(30): E7977-86. pdf
Kevin B. Dagbay, Nicolas Bolik-Coulon, Sergey N. Savinov and Jeanne A. Hardy (2017). “Caspase-6 Undergoes a Distinct Helix-Strand Interconversion Upon Substrate Binding” Journal of Biological Chemistry. 292: 4885-4897. pdf