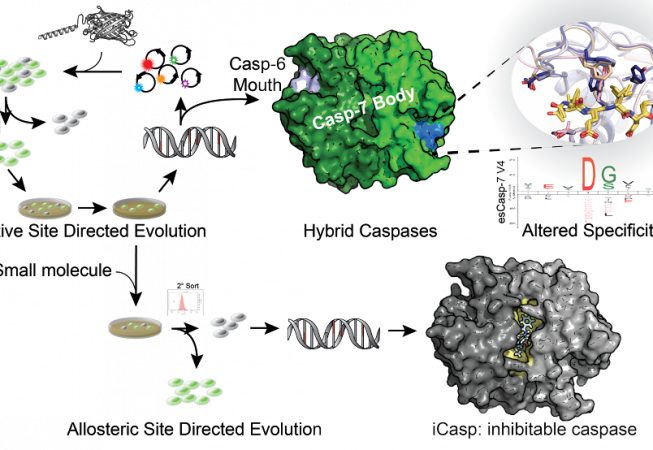
Our lab has developed a directed evolution method using our dark-to-bright GFP reporter that enables us to to tune specificity into intracellular proteases. In our first application of this technology, we sought to change the specificity of caspase-7 (DEVD) to that of caspase-6 (VEID) and produced evolved-specificity caspase-7 (esCasp-7) variants that maintained the body of caspase-7, but had caspase-6-like activity. With this hybrid enzyme, we sought to decipher proteins that may rely on non-active site interactions (exosites) for recognition. Such reprogrammed caspases represent a new method to discover exosite driven substrates and should be broadly applicable to many other proteases. We are also interested in engineering allosteric sites on caspases to develop iCasps: caspases that are inhibited by a small molecule of interest. This type of regulation is desirable so that caspase activity can be silenced only under specific cellular conditions.
Maureen E. Hill, Derek J. MacPherson, Peng Wu, Olivier Julien, James A. Wells, Jeanne A. Hardy, (2016). “Reprogramming Caspase-7 specificity to Caspase-6 by regio-specific mutations and selection” ACS Chemical Biology. 11(6):1603–1612. pdf
Samantha Nicholls and Jeanne A. Hardy, (2013). “Structural Basis of Fluorescence Quenching in Caspase Activatable-GFP.” Protein Science. 22(3), 247. Article featured as cover illustration. pdf
Samantha Nicholls, Jun Chu, Genevieve Abbruzzese, Kimberly D. Tremblay and Jeanne A. Hardy, (2011). “Mechanism of a dark-to-bright reporter of caspase activity.” Journal of Biological Chemistry, 286 (28), 24977-24986. pdf
Witold Witkowski and Jeanne A. Hardy, (2011). “A designed redox-controlled caspase.” Protein Science, 20, 1421-1431. Article featured as cover illustration. pdf